Adempas® – an sGC stimulator
A wide range of patients with PH can achieve everyday activities due to Adempas® and its direct stimulation of sGC independently of nitric oxide.1, 2 Adempas® is a distinct class of oral treatment. Adempas® demonstrates rapid, sustained and significant improvements in patients with inoperable, persistent or recurrent CTEPH, as well as PAH.3-6
Two large, international Phase III trials, CHEST-1 and PATENT-1, demonstrated the efficacy and tolerability of Adempas® in treating patients with inoperable, persistent or recurrent CTEPH or PAH, respectively.3,4 Patients with CTEPH or PAH, achieved rapid and sustained improvements across a broad range of clinically-important endpoints, enabling patients to achieve more everyday activities:1,3-6
Exercise capacity (as measured by improvement in six minute walk distance compared with placebo; the primary endpoint of both CHEST-1 and PATENT-1)
Overall disease severity (as measured by improvement in WHO functional class)
Cardiac output
Heart and lung function (as measured by NT-pro BNP, PVR and PAP)
Reduced breathlessness (as measured by improvements in Borg dyspnea score)
Time to clinical worsening (in PATENT-1)
The two-year extension studies, CHEST-2 and PATENT-2, showed that the benefits of Adempas® were sustained long-term.5,6 In patients with CTEPH or PAH, exercise capacity (as measured by 6MWD) and overall disease severity (as measured by WHO functional class) was either improved or sustained at two years.5,6 Additionally, Adempas® was well-tolerated by patients over the long-term with no new safety signals.5,6
Moreover, for patients with CTEPH or PAH, a total of 82% and 79%, respectively, of patients remained free from long-term clinical worsening and 93% remained alive after two years of treatment with Adempas®.5,6
Adempas® addresses areas of high unmet medical need
With Adempas®, patients with inoperable CTEPH, or persistent or recurrent CTEPH after surgical treatment, have a pharmacologic treatment that can improve their everyday possibilities.3,6 A wide range of patients with PAH, including treatment-naïve and pre-treated patients, can achieve rapid and sustained improvement across a broad range of endpoints using Adempas®.4,5 Adempas® is the first oral treatment to show significant and sustained efficacy as monotherapy or as part of a combination regimen with ERAs or prostanoids in patients with PAH.4, 7-11
Addressing different forms of PH underscores Bayer’s commitment to understanding this severe and life-threatening condition and help to improve the lives of people with PH.
Pulmonary hypertension
PH is a severe, progressive and life-threatening condition in which the pressure in the pulmonary arteries is significantly increased due to various mechanisms.12 It is defined as an elevated mean pulmonary arterial pressure of ≥ 25mmHg at rest.13
There are 5 main types of PH, all associated with a reduction in exercise capacity, increased arterial pressure and premature death.9,10
In PH, pulmonary vascular injury causes endothelial dysfunction and vascular remodeling through proliferation and hypertrophy of endothelial smooth muscle cells. This endothelial dysfunction causes vasoconstriction, inflammation and thrombosis which, in turn, results in the progressive increase in pulmonary vascular disease.14,15
In healthy individuals, nitric oxide plays a key role in blood vessel regulation
Endogenous nitric oxide, produced by the endothelial cells of blood vessels, is one of the factors that helps regulate vascular tone, proliferation and inflammation.16,17
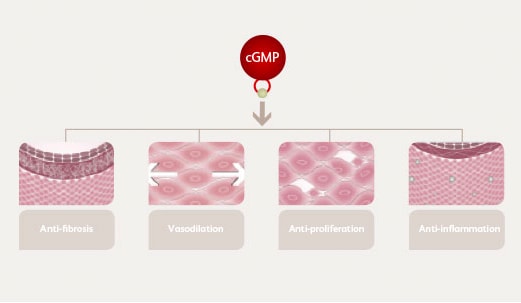
Nitric oxide binds to, and stimulates, sGC, a key enzyme that enhances the synthesis of a signaling molecule, cGMP. cGMP, in turn, has the following physiologic effects:17
induces vasorelaxation
inhibits smooth muscle proliferation, leukocyte recruitment and platelet aggregation
Together, these form the NO-sGC-cGMP pathway.16
Endothelial dysfunction associated with PH results in nitric oxide deficiency
Low levels of endogenous nitric oxide results in under-stimulation of sGC, which in turn causes decreased cGMP generation.14,17 Without cGMP, arterial walls tighten and thicken, and fibrosis, platelet aggregation, cell proliferation and inflammation occur.17
Patients with CTEPH or PAH are often nitric oxide deficient and this is associated with a poor prognosis.18,19
Adempas® directly stimulates sGC independently of nitric oxide and can restore the NO-sGC-cGMP pathway
Adempas® binds to and directly stimulates sGC independently of nitric oxide.1,2 Adempas® also facilitates the binding of any remaining endogenous nitric oxide to sGC in patients with inoperable, persistent or recurrent CTEPH or PAH.2
This may explain why Adempas® has such broad efficacy in terms of more than one type of PH and multiple clinical endpoints.
Watch the video to see how Adempas® works
References
- Adempas® Company Core Data Sheet, September 2013. Return to content
- Grimminger F et al. Eur Respir J 2009; 33:785–792. Return to content
- Ghofrani HA et al. New Engl J Med 2013; 369: 319-29. Return to content
- Ghofrani HA et al. New Engl J Med 2013; 369: 330-40. Return to content
- Ghofrani HA et al. Lancet Respir Med. 2016; 4: 361-71. Return to content
- Simonneau G, et al. Lancet Respir Med 2016; 4: 372–80. Return to content
- Galie N et al. Circulation 2008; 117: 3010-9. Return to content
- Pulido T et al. N Engl J Med 2013; 369: 809-18. Return to content
- Jais X al. J Am Coll Cardiol 2008; 52: 2127-34. Return to content
- Galie N et al. N Engl J Med 2005; 353: 2148-57. Return to content
- Rubin LJ et al. N Engl J Med 2002; 346: 896-903. Return to content
- Simmoneau G et al. J Am Coll Cardiol 2009; S43-S54. Return to content
- Galie N et al. Eur Heart J 2009; 30: 2493-537. Return to content
- Ghofrani HA et al. Future Cardiol 2010; 6:155-66. Return to content
- Farber HW et al. New Engl J Med 2004; 351: 1655-65. Return to content
- Boerrigter G, Burnett JC. Cardiovasc Drug Rev 2007; 25: 30-45. Return to content
- Stasch J-P et al. Circulation 2011; 123: 2263-73. Return to content
- Kielstein JT et al. Arterioscler Thromb Vasc Biol 2005; 25: 1414-18. Return to content
- Skoro-sajer N et al. Am J Respir Crit Care Med 2007; 176: 1154-60. Return to content






